Clinical Cooperations
We work closely together with our colleagues from the University Hospital of Regensburg. To foster scientific synergies and clinical translation the LIT supports four Clinical Cooperation Groups that address complementary clinical topics in immunomedicine. To this end, LIT scientists are embedded in these clinical cooperation groups and co-supervised by PIs of the LIT together with PIs of the Medical Faculty who are affiliated with the LIT. Discover more about our ground-breaking research in this truly cooperational format.

01 | Organ Transplantation | Professor Edward Geissler
Professor Geissler oversees a clinical cooperation research group engaged in Transplantation Immunology research. This group aims to develop strategies for reducing both the rejection of organ transplants and the need for conventional immunosuppressive drugs. To this end, Professor Geissler led an EU-FP7 international consortium known as The ONE Study—looking to apply immunomodulatory cellular therapy to renal transplant recipients to reduce allograft rejection. Additionally, he coordinated a clinical research group known as KFO 243—funded by the German Research Foundation (DFG)—which studied the early immunological determinants of late transplant outcome. New treatment strategies for reducing transplant-related pathology are expected to be clinically translated from this research.
Representing the Leibniz Institute for Immunotherapy, Professors Geissler and Hutchinson focus their combined efforts on the study of a regulatory macrophage (M regs) population. This shows promising therapeutic potential in humans, with two renal transplant recipients having already been treated with these cells (J Immunol, 187:2072-2078, 2011). Independently, Professor Hutchinson currently leads two EU-funded Marie Skłodowska-Curie Innovative Training Networks—researching regulatory monocyte-derived cells and the use of extracorporeal photophoresis to reduce alloimmune responses. Previously used to promote kidney allograft acceptance in The ONE Study, the two investigators are now engaged in research to determine how regulatory macrophages function in promoting tolerance to organ allografts. Both are additionally involved in a large integrated EU consortium (EU Pathfinder Challenge) on Emerging Technologies in Cell and Gene Therapy to develop a therapeutic tolerogenic DC cell product.
As part of this initiative, the following objectives have been identified:
Objective 1: Establish GMP-compliant, serum-free tissue culture conditions for manufacturing M regs
Objective 2: Identify a naturally occurring counterpart from the ex vivo-generated human M regs in tissue
Objective 3: Study the effect of M regs on co-cultured T cell and dendritic cell populations
Objective 4: Contribute to the establishment of an integrated immune-monitoring facility
Discover more by visiting Professor Geissler’s dedicated UKR page!
02 | Immunometabolomics | Professor Marina Kreutz
These days immunotherapies with checkpoint inhibitors and CAR T cells play a vital role in the treatment of cancer patients. That said, a variety of mechanisms by which solid tumors escape the immune system limit their therapeutic efficacy—with the metabolic tumor environment increasingly being attributed to T-cell therapy failure.
The Kreutz group is a pioneer in the field of immunometabolism, and was among the first to demonstrate T-cell immunosuppression triggered by lactic acid in the tumor micro environment. This occurs due to an accelerated tumor glucose metabolism known as the “immunological Warburg effect.” Additional research by the Immunometabolomics Clinical Cooperation Group has gone on to show a negative correlation between a high glycolytic index in melanoma patients’ biopsies and their response to checkpoint inhibition. Findings have shown that the genetic knockout of the lactate-producing enzyme leads to an increased response to checkpoint blockade in a murine melanoma model. A screen for drugs that stabilize T-cell function in this acidic environment reveals that the nonsteroidal anti-inflammatory drug ‘diclofenac’ inhibits lactate secretion and acidification—augmenting the response to checkpoint inhibition.
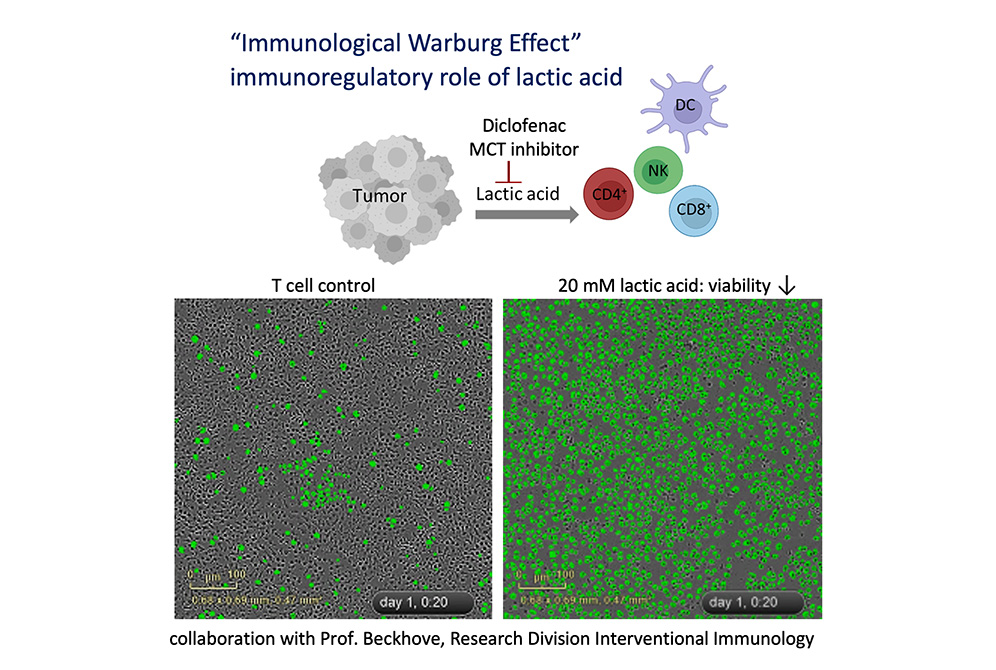
Based on these data a clinical trial has been initiated together with the Universities of Mainz and Essen. In this trial 'diclofenac' will be used to support checkpoint therapy (funding is focused on Translational Oncology provided by the German Cancer Aid [DKH]). Professor Marina Kreutz holds a patent (EP3104863) for the use of salicylates in the treatment of cancer and has filed a patent (DE102015103618) for the application of 'diclofenac' in cancer therapy.
In addition to this, the Immunometabolomics Clinical Cooperation Group is seeking to strengthen T cells and their resilience within this acidic tumor environment. In collaboration with the Core Facility (NGS & Data Technologies), RNAseq analyses of lactic acid-resistant T cells brought up interesting target genes. Together with Professor Simone Thomas (T-Cell Therapy Group) and Professor Hinrich Abken (Research Division Genetic Immunotherapy), they plan to equip T cells with additional genes to strengthen and support their efficacy in immunotherapeutic application.
Currently, Professor Kreutz is collaborating with Dr. Chi Van Dang (Ludwig Institute for Cancer Research, New York, USA) in order to deepen her investigations into metabolic targeting. Another ongoing collaboration with Merck KGaA, (Darmstadt, Germany) aims to further explore the impact of MCT inhibitors in the tumor environment.
Discover more by visiting Professor Kreutz’ dedicated UKR page!
03 | Inflammation, Autoimmunity & Fibrosis | Professor Matthias Mack
Professor Mack’s laboratory focuses on deciphering the role of different immune cells at a molecular level and their impact on disease and pathology. Of particular interest, is to understand the role of basophils, monocytes, and collagen-producing hematopoietic cells in autoimmunity, transplant rejection, and fibrosis. The ultimate goal of the team’s research is to develop new therapeutic approaches for clinical application. The following projects are currently underway:
Basophils as regulators of immune responses and fibrosis
Professor Mack’s team have identified previously obscure antigen-capturing cells as basophils. In preimmunized mice only two cell populations are able to bind (capture) significant amounts of antigen on their cell surface. These are; antigen-specific B cells that capture the antigen with the specific B cell receptor; and basophils that capture antigens using antigen-specific IgE bound to the high affinity FceRI-receptor. In mice the binding of antigens activates basophils to immediately release IL-4 and IL-6. During a secondary or memory immune response, basophils are responsible for the immediate production of IL-4 following the injection of the antigen. This leads to an enhancement of humoral memory immune responses. Basophils also constitute an important source of IL-4 in primary immune responses, as they can also be activated by various cytokines and other molecules like proteases from parasites. In conclusion, it is quite clear that basophils are an important source of the initial IL-4 which is required to induce a Th2 differentiation. By enhancing humoral and Th2 immune responses, basophils play a critical role in the development of diseases like rheumatoid arthritis and lupus nephritis. Moreover, due to the profibrotic properties of IL-4, basophils are also involved in fibrogenesis—as shown in chronic transplant rejection models. Going forward, work will continue into analyzing the role of basophils in autoimmunity and fibrosis.
The role of IL-3 in autoimmunity, inflammation, and fibrosis
IL-3 belongs to the family of hematopoietic cytokines which also include GM-CSF and IL-5. For many years IL-3 was considered to be mainly involved in hematopoiesis and defense against parasites. Professor Mack’s team have now identified IL-3 to be an essential cytokine in systemic lupus erythematodes (SLE), rheumatoid arthritis, multiple sclerosis, and fibrosis. Additionally, they have also proven that T cells are the main source of IL-3 in murine models of inflammation. Blockading IL-3 with an antibody or genetically deleting it altogether has been found to markedly improve disease activity in mouse models for systemic lupus (MRL-lpr), rheumatoid arthritis (collagen-induced arthritis, CIA), and multiple sclerosis (experimental autoimmune encephalitis, EAE). Along with others, the team have found that IL-3 is an essential inductor cytokine for several classical proinflammatory cytokines such as; IL-6, TNF-alpha, and IL-1. In addition, IL-3 is essential for the mobilization of innate immune cells in the bone marrow during inflammation. All in all, it is quite clear that the blockading of IL-3 interferes with multiple proinflammatory mechanisms. Reserach so far reveals that a blockade or deficiency of IL-3 is very well tolerated in mice. And that in contrast, an injection of recombinant IL-3 significantly increases disease activity in mouse models and induces polyarthritis in healthy rhesus monkeys.
In humans, IL-3 activates plasmocytoid dendritic cells (pDC), monocytes, basophils, mast cells, B cells, and endothelial cells. Plasmacytoid dendritic cells are the most important producers of type I interferons that play a central role in the pathogenesis of systemic lupus and also fibrosis. In endothelial cells, IL-3 induces the upregulation of E- and P-selectins—thus enabling the transendothelial migration of leukocytes. In the laboratory, the team have generated multiple monoclonal antibodies against human IL-3 and are currently analyzing the expression of IL-3 in patients with autoimmune and inflammatory diseases.

Fibrogenesis mechanisms with a focus on kidney and allograft fibrosis
Over recent years, the Institute's scientists and many others have been able to establish that collagen-producing cells in the kidney are derived from various cellular sources, including resident mesenchymal fibroblasts, pericytes, bone marrow-derived cells, tubular epithelial cells, and endothelial cells. Collagen-producing bone marrow-derived cells are frequently called fibrocytes. To this end, the team are investigating; the extent to which fibrocytes contribute to renal and allograft fibrosis; how they migrate into the organs; and how they are activated to produce collagen. Researchers are also investigating the functional relevance of cell-type-specific collagen production in the kidney and in cardiac allografts.
Functional analysis of monocyte subsets
Mouse and human monocytes can be divided into CCR2+ and CCR2- monocytes. The majority of monocytes comprise CCR2+ and are frequently found in inflamed tissue. Using a variety of animal models and antibodies to specifically deplete these cells, the LIT’s scientists and others have shown that CCR2+ monocytes contribute significantly to inflammation and tissue destruction. With this well proven, the team are currently developing new tools to specifically deplete CCR2+ monocytes in patients with inflammatory and autoimmune diseases.
Discover more by visiting Professor Mack’s dedicated UKR research page!
04 | Innate Immune Sensing in Cancer and Transplantation | Professor Hendrik Poeck
The central focus of Professor Poeck’s laboratory work is to identify the molecular mechanisms behind successful cancer therapies, as well as those found in therapy resistance. He and his team also look at tissue homeostatic responses during immunotherapeutic treatments such as allogeneic hematopoietic stem-cell transplantation (allo-HSCT), cellular therapies (chimeric antigen receptor [CAR] T cells or macrophages), immune checkpoint blockade (ICB), and certain chemotherapies. The specific aim is to understand how the innate immune system’s pattern recognition receptors (PRRs) can promote or inhibit these homeostatic responses in the tissues. Additionally they are investigating to what extent this is impacted by cellular engineering and environmental or cell-derived factors, such as the microbiome or extracellular vesicles.
The goal is then to develop new combinatorial strategies by gaining a deeper understanding of the modulation of anti-tumor and regenerative responses. These strategies will improve on existing cancer immunotherapies as they address the unsolved clinical problem of significant inter-individual response variability. At the same time they will ameliorate the damage done by unwanted immune-mediated tissue inflammation in conditions such as graft-versus-host disease (GvHD), cytokine-release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS by CAR T), and ICB-mediated autoinflammation.
A number of collaborations have been established here to promote further investigation. Close local cooperation with the following research groups has also been established: Meedt/Wer/Holler (translational GvHD and microbiome research); Edinger/Hoffmann (Treg cells); the Institutes of Pathology (Andreas Mamilos, Matthias Evert) and Microbiology and Hygiene (André Gessner, Andreas Hiergeist); Leibniz Institute for Immunotherapy (Hinrich Abken, Markus Feuerer, Christian Schmidl, Luca Gattinoni); as well as the Fraunhofer Institute ITEM (Melanie Werner Klein, Christian Werno).
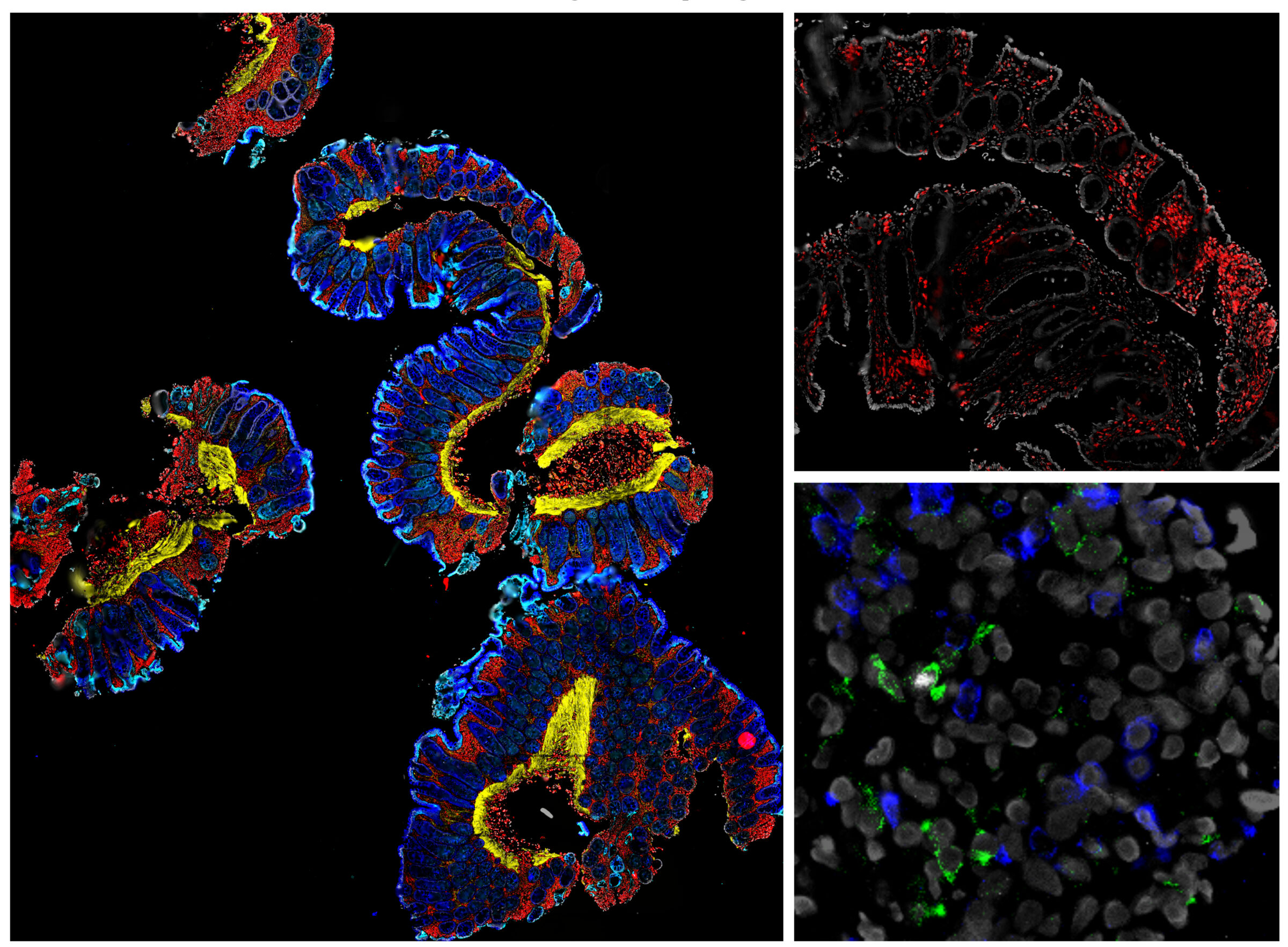
Colon biopsy samples using ChipCytometry. Epithelial markers include: Pan-cytokeratin, E-Cadherin, Vimentin, Vinculin. Lymphocytic markers include: CD3, CD4, CD8, Foxp3.
Recent focus: Modulation of CAR T-cell function by the microbiome
Cancer immunotherapy using CAR T cells has proved to be extraordinarily effective in treating hematological malignancies. Two such CAR T therapeutics, Kymriah (Tisagenlecleucel) and Yescarta (Axicabtagene ciloleucel), are now used to treat B-cell acute lymphoblastic leukemia (B-ALL) and diffuse large B-cell lymphoma (DLBCL). However, relapse rates of around 35% mean only a subgroup of patients will maintain long-term remission. In addition, challenges still remain for the use of CAR T-cell therapy in solid tumors.
The local microbial communities found in specific locations in the body are known as the bacterial microbiota. Recent studies have demonstrated that signals from the bacterial microbiota in the gastrointestinal (GI) tract, which indicate their composition, are linked to the outcome of patients receiving allo-HSCT as an immunotherapy against leukemias or lymphomas, as well as those receiving ICB treatment such as anti-CTLA-4 or PD1 inhibitors. Yet, the specific contribution of microbiota-mediated factors (e.g. metabolites) in CAR T-cell efficacy in hematologic and solid neoplasms remains unknown. In this project, Professor Poeck will analyze the role of microbiota-associated metabolites during CAR T-cell therapy in both mice and humans.
To this end, the team will utilize:
- genetically engineered tumor cell lines, specifically A20 (target: CD19), Nalm6 (target: CD19), CT26 (target: CEA or Cldn-6), and LL/2-LLc1 (target: CEA or Cldn-6) in syngeneic or humanized mouse models
- antimicrobial regimens (antibacterial/antifungal/antiviral)
- gnotobiotic mice as respective tumor recipients
- genetically modified immune cell (CAR T cells) transfer
- unbiased next-generation sequencing approaches
- targeted metabolomic profiling of stool samples, serum and intestinal tissues
Simultaneously, the team will investigate the relevance of microbiota-associated metabolites as potential biomarkers and predictors for developing anti-tumor responses, as well as for adverse events in human CAR T-cell recipients. This research will initially focus on hematologic cancers—such as ALL and lymphoma—and later on solid cancers—such as CEA+ colorectal carcinomas or Claudin-6 positive tumors.
Discover more by visiting Professor Poeck’s dedicated UKR page!
Selected Publications
Here is a selection of the most important publications from the last few years:
- Geissler EK, Kirste G, Meade MO, Chapman JR. (2023) Establishing Guidelines for Organ Donation Systems. Transplant Direct Apr 28;9(5):e1481. doi: 10.1097/TXD.0000000000001481. eCollection 2023 May.
- Schnitzbauer AA, Filmann N, Adam R, Bachellier P, Bechstein WO, Becker T, Bhoori S, Bilbao I, Brockmann J, Burra P, Chazoullières O, Cillo U, Colledan M, Duvoux C, Ganten TM, Gugenheim J, Heise M, van Hoek B, Jamieson N, de Jong KP, Klein CG, Klempnauer J, Kneteman N, Lerut J, Mäkisalo H, Mazzaferro V, Mirza DF, Nadalin S, Neuhaus P, Pageaux GP, Pinna AD, Pirenne J, Pratschke J, Powel J, Rentsch M, Rizell M, Rossi G, Rostaing L, Roy A, Scholz T, Settmacher U, Soliman T, Strasser S, Söderdahl G, Troisi RI, Turrión VS, Schlitt HJ, Geissler EK. (2020) mTOR inhibition is most beneficial after liver transplantation for Hepatocellular Carcinoma in patients with active tumors. Ann Surg. 272(5):855–862.
- Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, Tang Q, Guinan EC, Battaglia M, Burlingham WJ, Roberts ISD, Streitz M, Josien R, Böger CA, Scottà C, Markmann JF, Hester JL, Juerchott K, Braudeau C, James B, Contreras-Ruiz L, van der Net JB, Bergler T, Caldara R, Petchey W, Edinger M, Dupas N, Kapinsky M, Mutzbauer I, Otto NM, Öllinger R, Hernandez-Fuentes MP, Issa F, Ahrens N, Meyenberg C, Karitzky S, Kunzendorf U, Knechtle SJ, Grinyó J, Morris PJ, Brent L, Bushell A, Turka LA, Bluestone JA, Lechler RI, Schlitt HJ, Cuturi MC, Schlickeiser S, Friend PJ, Miloud T, Scheffold A, Secchi A, Crisalli K, Kang SM, Hilton R, Banas B, Blancho G, Volk HD, Lombardi G, Wood KJ, Geissler EK. (2020) Regulatory cell therapy in kidney Transplantation (The ONE Study): A harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet. 395(10237):1627-1639.
- Riquelme P, Haarer J, Kammler A, Walter L, Tomiuk S, Ahrens N, Wege AK, Goecze I, Zecher D, Banas B, Spang R, Fändrich F, Lutz MB, Sawitzki B, Schlitt HJ, Ochando J, Geissler EK, Hutchinson JA. (2018) TIGIT+ iTregs elicited by human regulatory macrophages control T cell immunity. Nat Commun. 9(1):2858.
- Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M, Ganten TM, Schmidt J, Settmacher U, Heise M, Rossi G, Cillo U, Kneteman N, Adam R, van Hoek B, Bachellier P, Wolf P, Rostaing L, Bechstein WO, Rizell M, Powell J, Hidalgo E, Gugenheim J, Wolters H, Brockmann J, Roy A, Mutzbauer I, Schlitt A, Beckebaum S, Graeb C, Nadalin S, Valente U, Sánchez Turrión V, Jamieson N, Scholz T, Colledan M, Fändrich F, Becker T, Söderdahl G, Chazouillères O, Mäkisalo H, Pageaux GP, Steininger R, Soliman T, de Jong KP, Pirenne J, Margreiter R, Pratschke J, Pinna AD, Hauss J, Schreiber S, Strasser S, Klempnauer J, Troisi RI, Bhoori S, Lerut J, Bilbao I, Klein CG, Königsrainer A, Mirza DF, Otto G, Mazzaferro V, Neuhaus P, Schlitt HJ. (2016) Sirolimus use in liver transplant recipients with hepatocellular carcinoma: A randomized, multicenter, open-label phase 3 trial. 100(1):116-125.
Here is a selection of the most important publications from the last few years:
- Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwartz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M. Inhibitory effect of tumor cell derived lactic acid on human T cells. Blood. 2007. doi: 10.1182/blood-2006-07-035972. (1726 citations)
- Gottfried E, Kunz-Schughart L, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006. doi: 10.1182/blood-2005-05-1795. (644 citations)
- Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, Kastenberger M, Bogdan C, Schleicher U, Mackensen A, Ullrich E, Fichtner-Feigl S, Kesselring R, Mack M, Ritter U, Schmid M, Blank C, Dettmer K, Oefner PJ, Hoffmann P, Walenta S, Geissler EK, Pouyssegur J, Villunger A, Steven A, Seliger B, Schreml S, Haferkamp S, Kohl E, Karrer S, Berneburg M, Herr W, Mueller-Klieser W, Renner K, Kreutz M. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016. doi: 10.1016/j.cmet.2016.08.011. (1173 citation)
- Comment in : Cell Metab. 2016 Nov 8;24(5):649-650. doi: 10.1016/j.cmet.2016.10.015, Lactate Wreaks Havoc on Tumor-Infiltrating T and NK Cells by Scott KE, Cleveland JL.
- Comment in: Nat Rev Immunol 2016 Oct 26;16(11):658-659. doi: 10.1038/nri.2016.119, Tumour immunology: Suppressive metabolites by Anna Dart
- Renner K, Bruss C, Schnell A, Koehl G, Becker H, Fante M, Menevse AN, Kauer N, Blazquez R, Hacker L, Decking SM, Bohn T, Faerber S, Evert K, Aigle L, Amslinger S, Landa M, Krijgsman O, Rozeman EA, Brummer C, Siska PJ, Singer K, Pektor S, Miederer M, Peter K, Gottfried E, Herr W, Marchiq I, Pouyssegur J, Roush WR, Ong S, Warren S, Pukrop T, Beckhove P, Lang SA, Bopp T, Blank CU, Cleveland JL, Oefner PJ, Dettmer K, Selby M, Kreutz M. Restricting Glycolysis Preserves T Cell Effector Functions and Augments Checkpoint Therapy. Cell Rep. 2019. doi: 10.1016/j.celrep.2019.08.068. (194 citations)
- Ždralević M, Brand A, Di Ianni L, Dettmer K,Reinders J, Singer K, Peter K, Schnell A, Bruss C, Decking SM, Koehl G,Felipe-Abrio B, Durivault J, Bayer P, Evangelista M, O’Brien T, Oefner PJ,Renner K, Pouysségur J, Kreutz M. Double genetic disruption of lactate dehydrogenases A and B is required to ablate the “Warburg effect” restricting tumor growth to oxidative metabolism. J BiolChem. 2018 Oct 12;293(41):15947-15961. (165 citations)
- Gottfried E, Lang SA, Renner K, Bosserhoff A, Gronwald W, Rehli M, Einhell S, Gedig I, Singer K, Seilbeck A, Mackensen A, Grauer O, Hau P, Dettmer K, Andreesen R, Oefner PJ, Kreutz M (2013). New Aspects of an Old Drug – Diclofenac Targets MYC and Glucose Metabolism in Tumor Cells. PLoS One. 2013 Jul 9;8(7). (105 citations)
Here is a selection of the most important publications from the last few years:
- Renner, K., T. Schwittay, S. Chaabane, J. Gottschling, C. Müller, C. Tiefenböck, J. N. Salewski, F. Winter, S. Buchtler, S. Balam, M. V. Malfertheiner, M. Lubnow, D. Lunz, B. Graf, F. Hitzenbichler, F. Hanses, H. Poeck, M. Kreutz, E. Orso, R. Burkhardt, T. Niedermair, C. Brochhausen, A. Gessner, B. Salzberger and M. Mack (2021). “Severe T cell hyporeactivity in ventilated COVID-19 patients correlates with prolonged virus persistence and poor outcomes.” Nat Commun 12(1): 3006.
- Buchtler, S., A. Grill, S. Hofmarksrichter, P. Stockert, G. Schiechl-Brachner, M. Rodriguez Gomez, S. Neumayer, K. Schmidbauer, Y. Talke, B. M. Klinkhammer, P. Boor, A. Medvinsky, K. Renner, H. Castrop and M. Mack (2018). “Cellular Origin and Functional Relevance of Collagen I Production in the Kidney.” J Am Soc Nephrol 29(7): 1859-1873.
- Renner, K., S. Hellerbrand, F. Hermann, C. Riedhammer, Y. Talke, G. Schiechl, M. R. Gomez, S. Kutzi, D. Halbritter, N. Goebel, H. Brühl, R. Weissert and M. Mack (2016). “IL-3 promotes the development of experimental autoimmune encephalitis.” JCI Insight 1(16): e87157.
- Schiechl, G., F. J. Hermann, M. Rodriguez Gomez, S. Kutzi, K. Schmidbauer, Y. Talke, S. Neumayer, N. Goebel, K. Renner, H. Bruhl, H. Karasuyama, K. Obata-Ninomiya, K. Utpatel, M. Evert, S. W. Hirt, E. K. Geissler, S. Fichtner-Feigl and M. Mack (2016). “Basophils Trigger Fibroblast Activation in Cardiac Allograft Fibrosis Development.” Am J Transplant 16(9): 2574-2588.
- Denzel, A., U. A. Maus, M. R. Gomez, C. Moll, M. Niedermeier, C. Winter, R. Maus, S. Hollingshead, D. E. Briles, L. A. Kunz-Schughart, Y. Talke and M. Mack (2008). “Basophils enhance immunological memory responses.” Nat Immunol 9(7): 733-742.
Here is a selection of the most important publications from the last few years:
- Heidegger S, Stritzke F, Dahl S, Daßler-Plenker J, Joachim L, Buschmann D, Winter C, Fan K, Enßle S, Li S, Perl M, Görgens A, Haas T, Thiele Orberg E, Göttert S, Wölfel C, Engleitner T, Rad R, Herr W, Giebel B, Ruland J, Bassermann F, Coch C, Hartmann G and Poeck H. Harnessing nucleic acid sensors in tumor cells to reprogram biogenesis and RNA cargo of extracellular vesicles for T-cell-mediated cancer immunotherapy, Cell Rep Med. 2023 Sep 19;4(9):101171.
- Thiele Orberg E, Meedt E, Hiergeist A, Xue J,Heinrich P, Ru J, Ghimire S, Miltiadous O, Lindner S, Tiefgraber M, Göldel S, Eismann T, Schwarz A, Göttert S, Jarosch S, Steiger K, Schulz C, Gigl M, Fischer JC, Janssen KP, Quante M, Heidegger S, Herhaus P, Verbeek M, Ruland J, van den Brink MRM, Weber D, Edinger M, Wolff D, Busch DH, Kleigrewe K, Herr W, Bassermann F, Gessner A, Deng L, Holler E, Poeck H. Bacterial and Bacteriophage Consortia are Associated with Protective Intestinal Immuno-modulatory Metabolites in Allogeneic Stem Cell Transplantation Patients [https://doi.org/10.21203/rs.3.rs-1504704/v1] (Nat Cancer 2023, in press)
- Heidegger S*#, Wintges A *, Stritzke F, Bek S, Steiger K, Koenig PA, Göttert S, Engleitner T, Öllinger R, Nedelko T, Fischer JC, Makarov V, Winter C, Rad R, van den Brink MRM, Ruland J, Bassermann F, Chan TA, Haas T*, Poeck H*#. RIG-I activation is critical for responsiveness to checkpoint blockade. Sci Immunol., 2019 Sep 13;4(39).
- Heidegger S*#, Kreppel D*, Bscheider M, Stritzke F, Nedelko T, Wintges A, Bek S,Fischer JC, Graalman T, Kalinke U, Bassermann F, Haas T*, Poeck H*#. RIG-I activating immunostimulatory RNA boosts the efficacy of anticancer vaccines and synergizes withcheckpoint blockade. EBioMedicine. 2019 Mar6. pii: S2352-3964(19)30135 [
- Fischer JC*, M Bscheider*, Gabriel Eisenkolb*, Lin CC, Wintges A, Otten V, Lindemans CA, Heidegger S, Rudelius M, Monette S, Porosnicu Rodriguez K, Calafiore M, Liebermann S, Liu C, Lienenklaus S, Weiss S, Kalinke U, Ruland J, Peschel C, Shono Y, Docampo M, Velardi E, Jenq R, Hanash A, Dudakov JA, Haas T, van den Brink MR *# and Poeck H*#. RIG-I/MAVS and STING signaling promote epithelial integrity during irradiation and immune-medited tissue injury; Sci Transl Med. 2017 Apr 19;9(386).
Visit the complete list of Prof. Poeck’s publications on PubMed:
https://pubmed.ncbi.nlm.nih.gov/?term=Poeck+H